Antibacterial ( antibiotics )
An antibacterial is a drug
that inhibits bacterial growth or kills bacteria. Synonymously
antibacterial is often used with the term antibiotic(s).
Antibiotics are any substance
produced by a microorganism that is antagonistic to the
growth of other microorganisms in high dilution. This definition excluded
substances that kill bacteria, but are not produced by microorganisms (such
as hydrogen
peroxide and gastric juices).
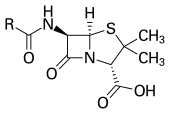
Most of today's antibacterials
chemically are semisynthetic modifications of various natural
compounds e.g. the beta-lactam antibacterials which include the penicillins, the cephalosporins, and the carbapenems.
Compounds that are still isolated
from living organisms are the aminoglycosides. Other
antibacterials e.g, the sulfonamides, the quinolones, and the oxazolidinones are produced solely by chemical
synthesis.
Many antibacterial compounds are
classified on the basis of biosynthetic/chemical origin
into natural, semisynthetic, and synthetic.
Another classification system is
based on biological activity were antibacterials are divided into two broad
groups according to their biological effect on microorganisms and they are:
- · bactericidal agents kill bacteria.
- bacteriostatic agents slow down or stall bacterial growth.
Discovery
of the first antibiotic:
Alexander Fleming discovered the first natural antibiotic (Penicillin) in
1928
Before the early 20th century,
treatments for infections were based primarily on medicinal folklore.
Mixtures with antimicrobial properties that were used in treatments of
infections were described over 2000 years ago. Many ancient cultures, including
the ancient
Egyptians and ancient Greeks, used specially selected mold and plant materials and extracts to treat infections. More
recent observations made in the laboratory of antibiosis between
micro-organisms led to the discovery of natural antibacterials produced by
microorganisms. Louis Pasteur observed, "if we could intervene in the antagonism
observed between some bacteria, it would offer perhaps the greatest hopes for therapeutics”.
Antibiosis was first described in 1877 in bacteria when Robert Koch and Louis Pasteur observed that an airborne bacillus
could inhibit the growth of Bacillus
anthracis.
John Tyndall first described antagonistic activities by fungi against
bacteria in England in 1875. Synthetic antibiotic chemotherapy as a science and
development of antibacterials began in Germany with Paul Ehrlich in the
late 1880s. Ehrlich noted certain dyes would color human, animal, or
bacterial cells. He then proposed the idea that it might be possible to create
chemicals that would act as a selective drug that would bind to and kill
bacteria without harming the human host. After screening hundreds of dyes
against various organisms, he discovered a medicinally useful drug, the
synthetic antibacterial Salvarsan now called arsphenamine.
In 1895, Vincenzo Tiberio discovered
that a mold (Penicillium)
in water well had an antibacterial action. In 1928, Alexander Fleming observed
that antibiosis against bacteria by a fungus of the genus Penicillium.
Fleming postulated the effect was mediated by an antibacterial compound named
penicillin, and that its antibacterial properties could be exploited for
chemotherapy. He initially characterized some of its biological properties, but
he did not pursue its further development.
Alexander Fleming
The first sulfonamide and first commercially available antibacterial Prontosil, was developed
by a research team led by Gerhard Domagk in 1932
at the Bayer Laboratories
of the IG Farben conglomerate in Germany. Domagk received the 1939 Nobel
Prize for Medicine for his
efforts. Prontosil had a relatively broad effect against Gram-positive cocci, but not
against enterobacteria.
In 1939, coinciding with the start of World
War II, Rene Dubos reported the discovery of the first naturally derived
antibiotic, gramicidin from B. brevis. It was one of the first commercially
manufactured antibiotics universally and very effectively used to treat wounds
and ulcers during World War II.
Florey and Chain succeeded in
purifying the first penicillin, penicillin G
procaine in 1942, but it did not become
widely available outside Allied military before 1945. The chemical structure of
penicillin was determined by Dorothy Crowfoot Hodgkin in 1945. Furthermore, its
activity was not inhibited by biological constituents such as pus, unlike the
synthetic sulfonamides. The discovery of such a powerful antibiotic was unprecedented,
and the development of penicillin led to renewed interest in the search for
antibiotic compounds with similar efficacy and safety. For their discovery and
development of penicillin as a therapeutic drug, Howard Florey , Alexander Fleming, and Ernst
Chain shared the 1945 Nobel Prize in
Medicine.
Medical
uses:
Treatment:
- Bacterial infection
- Protozoan infection, e.g., metronidazole is effective against several parasitics
- Prevention of infection
- Immunomodulation, e.g., tetracycline, which is effective in periodontal inflammation, and dapsone, which is effective in autoimmune diseases such as oral mucous membrane pemphigoid
- Dental antibiotic prophylaxis
- Surgical wound
- Conditions of neutropenia, e.g. cancer-related
Pharmacodynamics:
Testing the susceptibility of Staphylococcus
aureus to antibiotics by the Kirby-Bauer
disk diffusion method - antibiotics
diffuse from antibiotic containing disks and inhibit growth of S. aureus,
resulting in a zone called (zone of inhibition).
There are several factors that
define the successful outcome of antimicrobial therapy with antibacterial
compounds.
These factors include:
- Host defense mechanisms.
- The location of infection
- The pharmacokinetic and pharmacodynamic properties of the antibacterial.
- Bactericidal activity of antibacterials may depend on the bacterial growth phase, and it often requires ongoing metabolic activity and division of bacterial cells. These findings are based on laboratory studies, and in clinical settings have also been shown to eliminate bacterial infection. Since the activity of antibacterials depends frequently on its concentration, in vitro characterization of antibacterial activity commonly includes the determination of the minimum inhibitory concentration and minimum bactericidal concentration of an antibacterial.
Classes:
Molecular targets of antibiotics on the bacteria cell
Antibacterial
antibiotics are commonly classified based on:
- Their mechanism of action
- Chemical structure
- Spectrum of activity
Those that
target the bacterial cell wall:
(penicillins and cephalosporins)
or the cell membrane (polymixins), or interfere with essential bacterial enzymes
(rifamycins, lipiarmycins, quinolones,
and sulfonamides) have bactericidal activities.
Those that
target protein synthesis:
(macrolides, lincosamides and tetracyclines) are usually bacteriostatic (with the exception of bactericidal aminoglycosides). Further categorization is based on their target specificity. "Narrow-spectrum" antibacterial antibiotics target specific types of bacteria, such as Gram-negative or Gram-positive bacteria, whereas broad-spectrum antibiotics affect a wide range of bacteria. There are four new classes of antibacterial antibiotics have been brought into clinical use: cyclic lipopeptides (such as daptomycin), glycylcyclines (such as tigecycline),oxazolidinones (such as linezolid) and lipiarmycins (such as fidaxomicin).
(macrolides, lincosamides and tetracyclines) are usually bacteriostatic (with the exception of bactericidal aminoglycosides). Further categorization is based on their target specificity. "Narrow-spectrum" antibacterial antibiotics target specific types of bacteria, such as Gram-negative or Gram-positive bacteria, whereas broad-spectrum antibiotics affect a wide range of bacteria. There are four new classes of antibacterial antibiotics have been brought into clinical use: cyclic lipopeptides (such as daptomycin), glycylcyclines (such as tigecycline),oxazolidinones (such as linezolid) and lipiarmycins (such as fidaxomicin).
Production:
Since the first pioneering efforts of Florey and Chain in 1939, the importance of antibiotics, including antibacterials, to medicine has led to intense research into producing antibacterials at large scales. Following screening of antibacterials against a wide range of bacteria, production of the active compounds is carried out using fermentation, usually in strongly aerobic conditions.
Since the first pioneering efforts of Florey and Chain in 1939, the importance of antibiotics, including antibacterials, to medicine has led to intense research into producing antibacterials at large scales. Following screening of antibacterials against a wide range of bacteria, production of the active compounds is carried out using fermentation, usually in strongly aerobic conditions.
Administration:
Oral antibacterials are orally
ingested, whereas intravenous administration may be used in more serious cases, such as
deep-seated systemic
infections. Antibiotics may also sometimes be
administered topically, as with eye drops or ointments.
Side-effects:
Antibacterials are screened for any
negative effects on humans or other mammals before approval for clinical use,
and are usually considered safe and most are well tolerated. However, some
antibacterials have been associated with a range of adverse
effects. Side-effects range from mild to very serious depending on the
antibiotics used, the microbial organisms targeted, and the individual patient.
Safety profiles of newer drugs are often not as well established as for those
that have a long history of use. Adverse effects range from fever and
nausea to major allergic reactions, including photodermatitis and
anaphylaxis. Common side-effects include diarrhea, resulting
from disruption of the species composition in the intestinal flora,
resulting, for example, in overgrowth of pathogenic bacteria, such as Clostridium
difficile. Antibacterials can also
affect the vaginal flora, and may lead to overgrowth of yeast species of the genus Candida in the
vulvo-vaginal area. Additional side-effects can result from interaction with
other drugs, such as elevated risk of tendon damage from administration of a quinolone antibiotic
with a systemic corticosteroid. Some
scientists have hypothesized that the indiscriminate use of antibiotics alter
the host microbiota and this has been associated with chronic disease.
Drug-drug
interactions:
1.
Birth control pills
The majority of studies indicate
antibiotics do not interfere with contraceptive
pills, such as clinical studies that
suggest the failure rate of contraceptive pills caused by antibiotics is very
low (about 1%).In cases where antibacterials have been suggested to affect the
efficiency of birth control pills, such as for the broad-spectrum
antibacterial rifampicin, these cases may be due to an increase in the
activities of hepatic liver enzymes causing increased breakdown of the pill's
active ingredients. Effects on the intestinal flora, which might result in
reduced absorption of estrogens in the colon, have also been suggested, but
such suggestions have been inconclusive and controversial. Clinicians have
recommended that extra contraceptive measures be applied during therapies using
antibacterials that are suspected to interact with oral contraceptives.
2. Alcohol
Interactions between alcohol and
certain antibacterials may occur and may cause side-effects and decreased
effectiveness of antibacterial therapy.
"It is sensible to avoid
drinking alcohol when taking medication. However, it is unlikely that drinking
alcohol in moderation will cause problems if you are taking most common
antibiotics. However, there are specific types of antibiotics with which
alcohol should be avoided completely, because of serious side-effects."
Therefore, potential risks of
side-effects and effectiveness depend on the type of antibacterial
administered. Despite the lack of a categorical counterindication, the belief
that alcohol and antibacterials should never be mixed is widespread.
Antibacterial
such as : metronidazole, tinidazole, cephamandole, latamoxef, cefoperazone, cefmenoxime, and furazolidone, cause a disulfiram-like
chemical reaction with alcohol by inhibiting its breakdown by acetaldehyde
dehydrogenase, which may
result in vomiting, nausea, and shortness of breath.
Other effects of alcohol on
antibacterial activity include altered activity of the liver enzymes that break
down the antibacterial compound. In addition, serum levels of doxycycline
and erythromycin succinate two bacteriostatic antibacterials (see above)
may be reduced by alcohol consumption, resulting in reduced efficacy and
diminished pharmacotherapeutic effect.
Resistance:
SEM depicting methicillin-resistantStaphylococcus
aureus bacteria
The emergence of resistance of
bacteria to antibacterial drugs is a common phenomenon. Emergence of resistance
often reflects evolutionary processes that take place during antibacterial drug therapy.
The antibacterial treatment may select for bacterial strains with
physiologically or genetically enhanced capacity to survive high doses of
antibacterials. Under certain conditions, it may result in preferential growth
of resistant bacteria, while growth of susceptible bacteria is inhibited by the
drug. For example, antibacterial selection within whole bacterial
populations for strains having previously acquired antibacterial-resistance
genes was demonstrated in 1943 by the Luria–Delbrück
experiment. Survival of bacteria often
results from an inheritable resistance. Resistance to antibacterials also
occurs through horizontal gene
transfer. Horizontal transfer is more likely
to happen in locations of frequent antibiotic use. Antibacterials such as
penicillin and erythromycin, which used to have high efficacy against many
bacterial species and strains, have become less effective, because of increased
resistance of many bacterial strains. Antibacterial resistance may impose a
biological cost, thereby reducing fitness of resistant strains, which can limit the spread of
antibacterial-resistant bacteria, for example, in the absence of antibacterial
compounds. Additional mutations, however, may compensate for this fitness cost
and can aid the survival of these bacteria.
Antibiotics are natural products
produced by microorganism to compete against other microorganisms.
Paleontological data show that both antibiotics and antibiotics resistance are
ancient compounds and mechanisms. Natural antibiotics are evolutionarily
robust, i.e., microorganisms are often unable to develop resistance against
them. Molecular data confirm this observation, showing that the evolution of
bacterial proteins targeted by antibiotics is highly constrained compared with
the evolution of other proteins. For example, mutations in genes coding for
antibiotics-targeted proteins tend to be deleterious, making these genes
subject to strong purifying selection, which stringently maintains the sequence
and structure of their cognate proteins.
Several molecular mechanisms of
antibacterial resistance exist. Intrinsic antibacterial resistance may be part
of the genetic makeup of bacterial strains. For example, an antibiotic target
may be absent from the bacterial genome. Acquired resistance results from a mutation in the bacterial
chromosome or the acquisition of extra-chromosomal DNA. Antibacterial-producing
bacteria have evolved resistance mechanisms that have been shown to be similar
to, and may have been transferred to, antibacterial-resistant strains. The
spread of antibacterial resistance often occurs through vertical transmission
of mutations during growth and by genetic recombination of DNA by horizontal
genetic exchange. For
instance, antibacterial resistance genes can be exchanged between different
bacterial strains or species via plasmids that
carry these resistance genes. Plasmids that carry several different
resistance genes can confer resistance to multiple antibacterials. Cross-resistance
to several antibacterials may also occur when a resistance mechanism encoded by
a single gene conveys resistance to more than one antibacterial compound.
Antibacterial-resistant strains and
species, sometimes referred to as "superbugs", now contribute to the
emergence of diseases that were for a while well controlled. For example, emergent
bacterial strains causing tuberculosis (TB) that
are resistant to previously effective antibacterial treatments pose many
therapeutic challenges. Every year, nearly half a million new cases of multidrug-resistant
tuberculosis (MDR-TB)
are estimated to occur worldwide. For example, NDM-1 is a newly identified enzyme conveying bacterial resistance
to a broad range of beta-lactam antibacterials. The United
Kingdom's Health
Protection Agency has
stated that "most isolates with NDM-1 enzyme are resistant to all standard
intravenous antibiotics for treatment of severe infections."
Misuse
This poster from the U.S. Centers
for Disease Control and Prevention "Get Smart" campaign, intended for
use in doctors' offices and other healthcare facilities, warns that antibiotics
do not work for viral illnesses such as the common cold.
The first rule of antibiotics is try
not to use them, and the second rule is try not to use too many of them.
Inappropriate antibacterial
treatment and overuse of antibiotics have contributed to the emergence of
antibacterial-resistant bacteria. Self prescription of
antibacterials is an example of misuse. Many antibacterials are frequently
prescribed to treat symptoms or diseases that do not respond to antibacterial
therapy or are likely to resolve without treatment, or incorrect or suboptimal
antibacterials are prescribed for certain bacterial infections. The overuse of
antibacterials, like penicillin and erythromycin, have been associated with
emerging antibacterial resistance since the 1950s. Widespread usage of
antibacterial drugs in hospitals has also been associated with increases in
bacterial strains and species that no longer respond to treatment with the most
common antibacterials.
Common forms of antibacterial misuse
include excessive use of prophylactic antibiotics
in travelers and failure of medical professionals to prescribe the correct
dosage of antibacterials on the basis of the patient's weight and history of
prior use. Other forms of misuse include failure to take the entire prescribed
course of the antibacterial, incorrect dosage and administration, or failure to
rest for sufficient recovery. Inappropriate antibacterial treatment, for example,
is the prescription of antibacterials to treat viral infections such as
the common cold. One study on respiratory
tract infections found
"physicians were more likely to prescribe antibiotics to patients who
appeared to expect them".Multifactorial interventions aimed at both
physicians and patients can reduce inappropriate prescription of antibiotics.
Several organizations concerned with
antimicrobial resistance are lobbying to eliminate the unnecessary use of
antibacterials. The issues of misuse and overuse of antibiotics have been
addressed by the formation of the U.S. Interagency Task Force on Antimicrobial
Resistance. This task force aims to actively address antimicrobial resistance,
and is coordinated by the US Centers
for Disease Control and Prevention,
the Food and Drug
Administration (FDA),
and the National
Institutes of Health (NIH), as
well as other US agencies. An NGO campaign group is Keep Antibiotics
Working. In France, an "Antibiotics are not automatic"
government campaign started in 2002 and led to a marked reduction of
unnecessary antibacterial prescriptions, especially in children.
The emergence of antibacterial
resistance has prompted restrictions on antibacterial use in the UK in 1970
(Swann report 1969), and the EU has banned the use of antibacterials as
growth-promotional agents since 2003. Moreover, several organizations (e.g.,
The American Society for Microbiology (ASM), American Public Health Association
(APHA) and the American Medical Association (AMA)) have called for restrictions
on antibiotic use in food animal production and an end to all nontherapeutic
uses. However, commonly there are delays in regulatory and legislative actions
to limit the use of antibacterials, attributable partly to resistance against
such regulation by industries using or selling antibacterials, and to the time
required for research to test causal links between antibacterial use and
resistance. Two federal bills aimed at phasing out nontherapeutic use of
antibacterials in US food animals were proposed, but have not passed. These
bills were endorsed by public health and medical organizations, including the
American Holistic Nurses' Association, the American Medical Association, and
the American Public Health Association (APHA).
There has been extensive use of
antibiotics in animal husbandry. In the United States
the question of emergence
of antibiotic-resistant bacterial strains due to use of antibiotics in
livestock was raised by the United
States Food and Drug Administration in
1977. In March, 2012 the United States District Court for the Southern District
of New York, ruling in an action brought by the Natural
Resources Defense Council and
others, ordered the FDA to revoke approvals for the use of antibiotics in
livestock which violated FDA regulations.
Alternatives:
The increase in bacterial strains
that are resistant to conventional antibacterial therapies has prompted the
development of alternative strategies to treat bacterial diseases.
1.Resistance-modifying
agents
One strategy to address bacterial
drug resistance is the discovery and application of compounds that modify
resistance to common antibacterials. For example, some resistance-modifying
agents may inhibit multidrug resistance mechanisms, such as drug efflux from the
cell, thus increasing the susceptibility of bacteria to an antibacterial.
Targets include:
The efflux
inhibitor Phe-Arg-β-naphthylamide.
Beta-lactamase inhibitors, such
as clavulanic acid and sulbactam.
Metabolic stimuli such as sugar can
help eradicate a certain type of antibiotic tolerant bacteria by keeping their
metabolism active.
2.Phage therapy
Phage therapy is the use of viruses
that infect bacteria (i.e. phages) for the
treatment of bacterial infections. Phages are common in bacterial populations
and control the growth of bacteria in many environments, including in the
intestine, the ocean, and the soil. Phage therapy was in use in the 1920s and
1930s in the US, Western Europe, and Eastern Europe. However, success rates of
this therapy have not been firmly established, because only a limited number
of clinical trials testing the efficacy of phage therapy have been
conducted. These studies were performed mainly in the former Soviet Union,
at the Eliava Institute of Bacteriophage, Microbiology
and Virology, Republic of Georgia. The
development of antibacterial-resistant bacteria has sparked renewed interest in
phage therapy in Western medicine. Several companies (e.g., Intralytix,
Novolytics, and Gangagen), universities, and foundations across the world now
focus on phage therapies. One concern with this therapeutic strategy is the use
of genetically
engineered viruses, which limits certain
aspects of phage therapy.
3.Bacteriocins
Bacteriocins are peptides that can
be more readily engineered than small molecules, and are possible alternatives
to conventional antibacterial compounds. Different classes of bacteriocins
have different potential as therapeutic agents. Small-molecule bacteriocins (microcins and lantibiotics)
are similar to the classic antibiotics; colicin-like bacteriocins
possess a narrow spectrum, and require molecular diagnostics prior to
therapy. Limitations of large-molecule antibacterials include reduced
transport across membranes and within the human body. For this reason, they are
usually applied topically or gastrointestinally.
4.Chelation
Chelation of micronutrients that are
essential for bacterial growth to restrict pathogen spread in
vivo might supplement some antibacterials. For example, limiting the iron availability in the human body restricts bacterial
proliferation. Many bacteria, however, possess mechanisms (such as siderophores)
for scavenging iron within environmental niches in
the human body, and experimental developments of iron chelators, therefore, aim
to reduce iron availability specifically to bacterial pathogens.
5.Vaccines
Vaccines rely
on immune modulation
or augmentation. Vaccination either excites or reinforces the immune competency
of a host to ward off infection, leading to the activation of macrophages, the
production of antibodies, inflammation, and other
classic immune reactions. Antibacterial vaccines have been responsible for a
drastic reduction in global bacterial diseases. Vaccines made from attenuated
whole cells or lysates have been replaced largely by less reactogenic,
cell-free vaccines consisting of purified components, including capsular
polysaccharides and their conjugates, to protein carriers, as well as
inactivated toxins (toxoids) and proteins.
6.Biotherapy
Biotherapy may employ organisms,
such as protozoa, to consume the bacterial pathogens. Another such approach
is maggot therapy.
7.Probiotics
Probiotics consist of a live culture
of bacteria, which may become established as competing
symbionts, and inhibit or interfere with
colonization by microbial pathogens.
8.Host defense peptides
An additional therapeutic agent is
the enhancement of the multifunctional properties of natural anti-infectives,
such as cationic host defense (antimicrobial) peptides (HDPs).
9.Antimicrobial coatings
Functionalization
of antimicrobial surfaces can be
used for sterilization, self-cleaning, and surface protection.
10.Antimicrobial copper alloy surfaces
Copper-alloy surfaces have natural
intrinsic properties to effectively and quickly destroy bacteria. The United
States Environmental Protection Agency has
approved the registration of 355 different antibacterial
copper alloys that
kill E. coli, methicillin-resistant Staphylococcus
aureus Staphylococcus, Enterobacter
aerogenes, and Pseudomonas aeruginosa in less than 2 hours of
contact. As a public hygienic measure in addition to regular cleaning, antimicrobial
copper alloys are being
installed in healthcare facilities and in a subway transit system.
References:

.jpg/200px-Staphylococcus_aureus_(AB_Test).jpg)









0 comments:
Post a Comment