Biotechnology
Biotechnology is using of living systems and organisms to make or develop useful products, or "using of biological systems, living organisms or derivatives through any technological application thereof, to modify or make products or processes for specific use". It often overlaps with the (related) fields of bioengineering and biomedical engineering depending on the tools and applications,
For thousands of years, humankind has used biotechnology in food production, agriculture and medicine. The term itself is largely believed to have been coined in 1919 by Hungarian engineer Karl Ereky. In the late 20th and early 21st century, biotechnology has expanded to include new and diverse sciences such as genomics, recombinant gene technologies, applied immunology, and development of pharmaceutical therapies and diagnostic tests.
Insulin crystals
Definitions of biotechnology:
The concept of 'biotech' or 'biotechnology' encompasses a wide range of procedures (and history) for modifying living organisms according to human purposes — going back to domestication of animals, cultivation of plants, and "improvements" to these through breeding programs that employ artificial selection and hybridization. Modern usage also includes genetic engineering as well as cell and tissue culture technologies. Biotechnology is defined by the American Chemical Society as the application of biological organisms, systems, or processes by various industries to learning about the science of life and the improvement of the value of materials and organisms such as pharmaceuticals, crops, and livestock. In other words, biotechnology can be defined as the mere application of technical advances in life science to develop commercial products.
Biotechnology also draws on the pure biological sciences (genetics, microbiology, animal cell culture, molecular biology, biochemistry, embryology, cell biology). And in many instances it is also dependent on knowledge and methods from outside the sphere of biology including:
- chemical engineering
- bioprocess engineering
- bioinformatics, a new brand of information technology
- biorobotics
Conversely, modern biological sciences (including even concepts such as molecular ecology) are intimately entwined and heavily dependent on the methods developed through biotechnology and what is commonly thought of as the life sciences industry. Biotechnology is the research and development in the laboratory using bioinformatics for exploration, extraction, exploitation and production from any living organisms and any source of biomass by means of biochemical engineering where high value-added products could be planned (reproduced by biosynthesis, for example), forecasted, formulated, developed, manufactured and marketed for the purpose of sustainable operations (for the return from bottomless initial investment on R & D) and gaining durable patents rights (for exclusives rights for sales, and prior to this to receive national and international approval from the results on animal experiment and human experiment, especially on the pharmaceutical branch of biotechnology to prevent any undetected side-effects or safety concerns by using the products).
By contrast, bioengineering is generally thought of as a related field with its emphasis more on higher systems approaches (not necessarily altering or using biological materials directly) for interfacing with and utilizing living things. Bioengineering is the application of the principles of engineering and natural sciences to tissues, cells and molecules . This can be considered as the use knowledge from working with and manipulating biology to achieve a result that can improve functions in plants and animals. Relatedly, biomedical engineering is an overlapping field that often draws upon and applies biotechnology (by various definitions), especially in certain sub-fields of biomedical and/or chemical engineering such as tissue engineering, biopharmaceutical engineering, and genetic engineering.
History of Biotechnology:
Brewing was an early application of biotechnology
Although not normally what first comes to mind, many forms of human-derived agriculture clearly fit the broad definition of "using a biotechnological system to make products". Indeed, the cultivation of plants may be viewed as the earliest biotechnological enterprise.
Agriculture has been theorized to have become the dominant way of producing food since the Neolithic Revolution. Through early biotechnology, the earliest farmers selected and bred the best suited crops, having the highest yields, to produce enough food to support a growing population. As crops and fields became increasingly large and difficult to maintain, it was discovered that specific organisms and their by-products could effectively fertilize, restore nitrogen, and control pests. Throughout the history of agriculture, farmers have inadvertently altered the genetics of their crops through introducing them to new environments and breeding them with other plants --- one of the first forms of biotechnology.
These processes also were included in early fermentation of beer. These processes were introduced in early Mesopotamia, Egypt, China and India, and still use the same basic biological methods. In brewing, malted grains (containing enzymes) convert starch from grains into sugar and then adding specific yeasts to produce beer. In this process, carbohydrates in the grains were broken down into alcohols such as ethanol. Later other cultures produced the process of lactic acid fermentation which allowed the fermentation and preservation of other forms of food, such as soy sauce. Fermentation was also used in this time period to produce leavened bread. Although the process of fermentation was not fully understood until Louis Pasteur's work in 1857, it is still the first use of biotechnology to convert a food source into another form.
For thousands of years, humans have used selective breeding to improve production of crops and livestock to use them for food. In selective breeding, organisms with desirable characteristics are mated to produce offspring with the same characteristics. For example, this technique was used with corn to produce the largest and sweetest crops.
In the early twentieth century scientists gained a greater understanding of microbiology and explored ways of manufacturing specific products. In 1917, Chaim Weizmann first used a pure microbiological culture in an industrial process, that of manufacturing corn starch using Clostridium acetobutylicum, to produce acetone, which the United Kingdom desperately needed to manufacture explosives during World War I.
Biotechnology has also led to the development of antibiotics. In 1928, Alexander Fleming discovered the mold Penicillium. His work led to the purification of the antibiotic by Howard Florey, Ernst Boris Chain and Norman Heatley, penicillin. In 1940, penicillin became available for medicinal use to treat bacterial infections in humans.
The field of modern biotechnology is generally thought of as having been born in 1971 when Paul Berg's (Stanford) experiments in gene splicing had early success. Herbert W. Boyer (Univ. Calif. at San Francisco) and Stanley N. Cohen (Stanford) significantly advanced the new technology in 1972 by transferring genetic material into a bacterium, such that the imported material would be reproduced. The commercial viability of a biotechnology industry was significantly expanded on June 16, 1980, when the United States Supreme Court ruled that a genetically modified microorganism could be patented in the case of Diamond v. Chakrabarty. Indian-born Ananda Chakrabarty, working for General Electric, had modified a bacterium (of the Pseudomonas genus) capable of breaking down crude oil, which he proposed to use in treating oil spills. (Chakrabarty's work did not involve gene manipulation but rather the transfer of entire organelles between strains of the Pseudomonas bacterium.
Revenue in the industry is expected to grow by 12.9% in 2008. Another factor influencing the biotechnology sector's success is improved intellectual property rights legislation—and enforcement—worldwide, as well as strengthened demand for medical and pharmaceutical products to cope with an ageing, and ailing, U.S. population.
Rising demand for biofuels is expected to be good news for the biotechnology sector, with the Department of Energy estimating ethanol usage could reduce U.S. petroleum-derived fuel consumption by up to 30% by 2030. The biotechnology sector has allowed the U.S. farming industry to rapidly increase its supply of corn and soybeans—the main inputs into biofuels—by developing genetically modified seeds which are resistant to pests and drought. By boosting farm productivity, biotechnology plays a crucial role in ensuring that biofuel production targets are met.
Biotechnology has applications in four major industrial areas, including health care (medical), crop production and agriculture, non food (industrial) uses of crops and other products (e.g. biodegradable plastics, vegetable oil, biofuels), and environmental uses.
For example, one application of biotechnology is the directed use of organisms for the manufacture of organic products (examples include beer and milk products). Another example is using naturally present bacteria by the mining industry in bioleaching. Biotechnology is also used to recycle, treat waste, cleanup sites contaminated by industrial activities (bioremediation), and also to produce biological weapons.
A series of derived terms have been coined to identify several branches of biotechnology; for example:
- Bioinformatics is an interdisciplinary field which addresses biological problems using computational techniques, and makes the rapid organization and analysis of biological data possible. The field may also be referred to as computational biology, and can be defined as, "conceptualizing biology in terms of molecules and then applying informatics techniques to understand and organize the information associated with these molecules, on a large scale." Bioinformatics plays a key role in various areas, such as functional genomics, structural genomics, and proteomics, and forms a key component in the biotechnology and pharmaceutical sector.
- Blue biotechnology is a term that has been used to describe the marine and aquatic applications of biotechnology, but its use is relatively rare.
- Green biotechnology is biotechnology applied to agricultural processes. An example would be the selection and domestication of plants via micropropagation. Another example is the designing of transgenic plants to grow under specific environments in the presence (or absence) of chemicals. One hope is that green biotechnology might produce more environmentally friendly solutions than traditional industrial agriculture. An example of this is the engineering of a plant to express a pesticide, thereby ending the need of external application of pesticides. An example of this would be Bt corn. Whether or not green biotechnology products such as this are ultimately more environmentally friendly is a topic of considerable debate.
- Red biotechnology is applied to medical processes. Some examples are the designing of organisms to produce antibiotics, and the engineering of genetic cures through genetic manipulation.
- White biotechnology, also known as industrial biotechnology, is biotechnology applied to industrial processes. An example is the designing of an organism to produce a useful chemical. Another example is the using of enzymes as industrial catalysts to either produce valuable chemicals or destroy hazardous/polluting chemicals. White biotechnology tends to consume less in resources than traditional processes used to produce industrial goods.
The investment and economic output of all of these types of applied biotechnologies is termed as bioeconomy.
A rose plant that began as cells grown in a tissue culture
Medicine:
In medicine, modern biotechnology finds promising applications in such areas as:- drug production.
- pharmacogenomics.
- gene therapy.
- genetic testing (or genetic screening): techniques in molecular biology detect genetic diseases. To test the developing fetus for Down syndrome, Amniocentesis and chorionic villus sampling can be used.
Pharmacogenomics:
Pharmacogenomics is the study of how the genetic inheritance of an individual affects his/her body's response to drugs. It is a compound derived from the root of the word "pharmacology" plus the word "genomics". It is hence the study of the relationship between pharmaceuticals and genetics.
The vision of pharmacogenomics is to be able to design and produce drugs that are adapted to each person's genetic makeup
.
Pharmacogenomics results in the following benefits:
1) Development of tailor-made medicines. Using pharmacogenomics, pharmaceutical companies can create drugs based on the proteins, enzymes and RNA molecules that are associated with specific genes and diseases. These tailor-made drugs promise not only to maximize therapeutic effects but also to decrease damage to nearby healthy cells.
2) More accurate methods of determining appropriate drug dosages. Knowing a patient's genetics will enable doctors to determine how well his/ her body can process and metabolize a medicine. This will maximize the value of the medicine and decrease the likelihood of overdose.
3) Improvements in the drug discovery and approval process. The discovery of potential therapies will be made easier using genome targets. Genes have been associated with numerous diseases and disorders. With modern biotechnology, these genes can be used as targets for the development of effective new therapies, which could significantly shorten the drug discovery process.
4) Better vaccines. Safer vaccines can be designed and produced by organisms transformed by means of genetic engineering. These vaccines will elicit the immune response without the attendant risks of infection. They will be inexpensive, stable, easy to store, and capable of being engineered to carry several strains of pathogen at once.
DNA microarray chip – some can do as many as a million blood tests at once
Pharmaceutical products:
Computer-generated image of insulin hexamers highlighting the threefold symmetry, the zinc ions holding it together, and the histidine residues involved in zinc binding.
Most traditional pharmaceutical drugs are relatively small molecules that bind to particular molecular targets and either activate or deactivate biological processes. Small molecules are typically manufactured through traditional organic synthesis, and many can be taken orally. In contrast, Biopharmaceuticals are large biological molecules such as proteins that are developed to address targets that cannot easily be addressed by small molecules. Some examples of biopharmaceutical drugs include Infliximab, a monoclonal antibody used in the treatment of autoimmune diseases, Etanercept, a fusion protein used in the treatment of autoimmune diseases, and Rituximab, a chimeric monoclonal antibody used in the treatment of cancer. Due to their larger size, and corresponding difficulty with surviving the stomach, colon and liver, biopharmaceuticals are typically injected.
Modern biotechnology is often associated with the use of genetically altered microorganisms such as E. coli or yeast for the production of substances like synthetic insulin or antibiotics. It can also refer to transgenic animals or transgenic plants, such as Bt corn. Genetically altered mammalian cells, such as Chinese Hamster Ovary cells (CHO), are also used to manufacture certain pharmaceuticals. Another promising new biotechnology application is the development of plant-made pharmaceuticals.
Biotechnology is also commonly associated with landmark breakthroughs in new medical therapies to treat hepatitis B, hepatitis C, cancers, arthritis, haemophilia, bone fractures, multiple sclerosis, and cardiovascular disorders. The biotechnology industry has also been instrumental in developing molecular diagnostic devices that can be used to define the target patient population for a given biopharmaceutical. Herceptin, for example, was the first drug approved for use with a matching diagnostic test and is used to treat breast cancer in women whose cancer cells express the protein HER2.
Modern biotechnology can be used to manufacture existing medicines relatively easily and cheaply. The first genetically engineered products were medicines designed to treat human diseases. To cite one example, in 1978 Genentech developed synthetic humanized insulin by joining its gene with a plasmid vector inserted into the bacterium Escherichia coli. Insulin, widely used for the treatment of diabetes, was previously extracted from the pancreas of abattoir animals (cattle and/or pigs). The resulting genetically engineered bacterium enabled the production of vast quantities of synthetic human insulin at relatively low cost. According to a 2003 study undertaken by the International Diabetes Federation (IDF) on the access to and availability of insulin in its member countries, synthetic 'human' insulin is considerably more expensive in most countries where both synthetic 'human' and animal insulin are commercially available: e.g. within European countries the average price of synthetic 'human' insulin was twice as high as the price of pork insulin. Yet in its position statement, the IDF writes that "there is no overwhelming evidence to prefer one species of insulin over another" and "[modern, highly purified] animal insulins remain a perfectly acceptable alternative.
Modern biotechnology has evolved, making it possible to produce more easily and relatively cheaply human growth hormone, clotting factors for hemophiliacs, fertility drugs, erythropoietin and other drugs. Most drugs today are based on about 500 molecular targets. Genomic knowledge of the genes involved in diseases, disease pathways, and drug-response sites are expected to lead to the discovery of thousands more new targets.
Computer-generated image of insulin hexamers highlighting the threefold symmetry, the zinc ions holding it together, and the histidine residues involved in zinc binding.
Genetic testing:
Genetic testing involves the direct examination of the DNA molecule itself. A scientist scans a patient's DNA sample for mutated sequences.
There are two major types of gene tests. In the first type, a researcher may design short pieces of DNA ("probes") whose sequences are complementary to the mutated sequences. These probes will seek their complement among the base pairs of an individual's genome. If the mutated sequence is present in the patient's genome, the probe will bind to it and flag the mutation. In the second type, a researcher may conduct the gene test by comparing the sequence of DNA bases in a patient's gene to disease in healthy individuals or their progeny.
Genetic testing is now used for:
- Carrier screening, or the identification of unaffected individuals who carry one copy of a gene for a disease that requires two copies for the disease to manifest
- Confirmational diagnosis of symptomatic individuals
- Determining sex
- Forensic/identity testing
- Newborn screening
- Prenatal diagnostic screening
- Presymptomatic testing for estimating the risk of developing adult-onset cancers
- Presymptomatic testing for predicting adult-onset disorders
Some genetic tests are already available, although most of them are used in developed countries. The tests currently available can detect mutations associated with rare genetic disorders like cystic fibrosis, sickle cell anemia, and Huntington's disease.
Recently, tests have been developed to detect mutation for a handful of more complex conditions such as breast, ovarian, and colon cancers. However, gene tests may not detect every mutation associated with a particular condition because many are as yet undiscovered.
Gel electrophoresis
Controversial questions:
The bacterium Escherichia coli is routinely genetically engineered.
The absence of privacy and anti-discrimination legal protections in most countries can lead to discrimination in employment or insurance or other use of personal genetic information. This raises questions such as whether genetic privacy is different from medical privacy.
1) Reproductive issues. These include the use of genetic information in reproductive decision-making and the possibility of genetically altering reproductive cells that may be passed on to future generations. For example, germline therapy changes the genetic make-up of an individual's descendants. Thus, any error in technology or judgment may have far-reaching consequences (though the same can also happen through natural reproduction). Ethical issues like designed babies and human cloning have also given rise to controversies between and among scientists and bioethicists, especially in the light of past abuses with eugenics.
2) Clinical issues. These center on the capabilities and limitations of doctors and other health-service providers, people identified with genetic conditions, and the general public in dealing with genetic information.
3) Effects on social institutions. Genetic tests reveal information about individuals and their families. Thus, test results can affect the dynamics within social institutions, particularly the family.
4) Conceptual and philosophical implications regarding human responsibility, free will vis-à-vis genetic determinism, and the concepts of health and disease.
The bacterium Escherichia coli is routinely genetically engineered.
Gene therapy:
Gene therapy using an Adenovirus vector. A new gene is inserted into an adenovirus vector, which is used to introduce the modified DNA into a human cell. If the treatment is successful, the new gene will make a functional protein.
Gene therapy may be used for treating, or even curing, genetic and acquired diseases like cancer and AIDS by using normal genes to supplement or replace defective genes or to bolster a normal function such as immunity. It can be used to target somatic cells (i.e., those of the body) or gamete (i.e., egg and sperm) cells. In somatic gene therapy, the genome of the recipient is changed, but this change is not passed along to the next generation. In contrast, in germline gene therapy, the egg and sperm cells of the parents are changed for the purpose of passing on the changes to their offspring.
There are basically two ways of implementing a gene therapy treatment:
1) Ex vivo, which means "outside the body" – Cells from the patient's blood or bone marrow are removed and grown in the laboratory. They are then exposed to a virus carrying the desired gene. The virus enters the cells, and the desired gene becomes part of the DNA of the cells. The cells are allowed to grow in the laboratory before being returned to the patient by injection into a vein.
2) In vivo, which means "inside the body" – No cells are removed from the patient's body. Instead, vectors are used to deliver the desired gene to cells in the patient's body.
As of June 2001, more than 500 clinical gene-therapy trials involving about 3,500 patients have been identified worldwide. Around 78% of these are in the United States, with Europe having 18%. These trials focus on various types of cancer, although other multigenic diseases are being studied as well. Recently, two children born with severe combined immunodeficiency disorder ("SCID") were reported to have been cured after being given genetically engineered cells.
Gene therapy faces many obstacles before it can become a practical approach for treating disease. At least four of these obstacles are as follows:
- Gene delivery tools. Genes are inserted into the body using gene carriers called vectors. The most common vectors now are viruses, which have evolved a way of encapsulating and delivering their genes to human cells in a pathogenic manner. Scientists manipulate the genome of the virus by removing the disease-causing genes and inserting the therapeutic genes. However, while viruses are effective, they can introduce problems like toxicity, immune and inflammatory responses, and gene control and targeting issues. In addition, in order for gene therapy to provide permanent therapeutic effects, the introduced gene needs to be integrated within the host cell's genome. Some viral vectors effect this in a random fashion, which can introduce other problems such as disruption of an endogenous host gene.
- High costs. Since gene therapy is relatively new and at an experimental stage, it is an expensive treatment to undertake. This explains why current studies are focused on illnesses commonly found in developed countries, where more people can afford to pay for treatment. It may take decades before developing countries can take advantage of this technology.
- Limited knowledge of the functions of genes. Scientists currently know the functions of only a few genes. Hence, gene therapy can address only some genes that cause a particular disease. Worse, it is not known exactly whether genes have more than one function, which creates uncertainty as to whether replacing such genes is indeed desirable.
- Multigene disorders and effect of environment. Most genetic disorders involve more than one gene. Moreover, most diseases involve the interaction of several genes and the environment. For example, many people with cancer not only inherit the disease gene for the disorder, but may have also failed to inherit specific tumor suppressor genes. Diet, exercise, smoking and other environmental factors may have also contributed to their disease.
Gene therapy using an Adenovirus vector. A new gene is inserted into an adenovirus vector, which is used to introduce the modified DNA into a human cell. If the treatment is successful, the new gene will make a functional protein.
Human Genome Project:
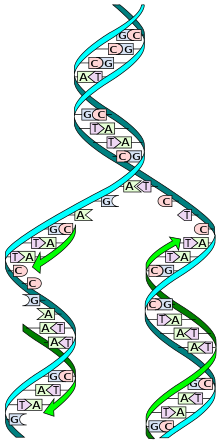
DNA Replication image from the Human Genome Project (HGP)
The Human Genome Project is an initiative of the U.S. Department of Energy ("DOE") and the National Institutes of Health ("NIH") that aims to generate a high-quality reference sequence for the entire human genome and identify all the human genes.
The DOE and its predecessor agencies were assigned by the U.S. Congress to develop new energy resources and technologies and to pursue a deeper understanding of potential health and environmental risks posed by their production and use. In 1986, the DOE announced its Human Genome Initiative. Shortly thereafter, the DOE and National Institutes of Health developed a plan for a joint Human Genome Project ("HGP"), which officially began in 1990.
The HGP was originally planned to last 15 years. However, rapid technological advances and worldwide participation accelerated the completion date to 2003 (making it a 13 year project). Already it has enabled gene hunters to pinpoint genes associated with more than 30 disorders.
Cloning:
Cloning involves the removal of the nucleus from one cell and its placement in an unfertilized egg cell whose nucleus has either been deactivated or removed.
There are two types of cloning:
1) Reproductive cloning. After a few divisions, the egg cell is placed into a uterus where it is allowed to develop into a fetus that is genetically identical to the donor of the original nucleus.
2) Therapeutic cloning. The egg is placed into a Petri dish where it develops into embryonic stem cells, which have shown potentials for treating several ailments.
In February 1997, cloning became the focus of media attention when Ian Wilmut and his colleagues at the Roslin Institute announced the successful cloning of a sheep, named Dolly, from the mammary glands of an adult female. The cloning of Dolly made it apparent to many that the techniques used to produce her could someday be used to clone human beings. This stirred a lot of controversy because of its ethical implications.
Agriculture:
- Crop yield:
Using the techniques of modern biotechnology, one or two genes (Smartstax from Monsanto in collaboration with Dow AgroSciences will use eight, starting in 2010) may be transferred to a highly developed crop variety to impart a new character that would increase its yield. However, while increases in crop yield are the most obvious applications of modern biotechnology in agriculture, they are also the most difficult ones. Current genetic engineering techniques work best for effects that are controlled by a single gene. Many of the genetic characteristics associated with yield (e.g., enhanced growth) are controlled by a large number of genes, each of which has a minimal effect on the overall yield. There is, therefore, much scientific work to be done in this area.
- Reduced vulnerability of crops to environmental stresses:
Crops containing genes that will enable them to withstand biotic and abiotic stresses may be developed. For example, drought and excessively salty soil are two important limiting factors in crop productivity. Biotechnologists work to find genes that enable some plants to cope with these extreme conditions and eventually to transfer these genes to the more productive crops. One of the latest developments is the identification of a plant gene, At-DBF2, from Arabidopsis thaliana. Arabidopsis thaliana is a tiny weed often used for plant research because it is very easy to grow. Its genetic code, approximately 115 Mb of the 125 Mb genome, which has been sequenced and interpreted and which can be manipulated in many ways. The At-DBF2 gene shows tolerance to salt, drought and the heat and cold in plants. When this gene was inserted into tomato and tobacco cells (see RNA interference), the cells withstood these conditions far better than ordinary cells. If these preliminary results prove successful in larger trials, then At-DBF2 genes can help in engineering crops that can better withstand harsh environments. Researchers have also created transgenic rice plants that resist rice yellow mottle virus (RYMV). In Africa, this virus destroys a majority of the rice crops and makes the surviving plants more susceptible to fungal infections. While all of these technological advances have the probability for commercial use, they need to be researched more publicly so they can be proven as a stable source of production.
- Increased nutritional qualities:
Proteins in foods may be modified to increase their nutritional qualities. Proteins in legumes and cereals may be transformed to provide the amino acids needed by human beings for a balanced diet. An example is the work of Professors Ingo Potrykus and Peter Beyer in creating Golden rice. The rice was a result of utilizing genetic modification with genetic material from corn and a soil microorganism. The genetically modified rice produced beta carotene which is converted to vitamin A. The extra beta carotene content turned the rice a golden color.
- Improved taste, texture or appearance of food:
Modern biotechnology can be used to slow down the process of spoilage. Modified fruit can ripen longer on the plant and then be transported to the consumer with less risk of spoilage, and a still-reasonable shelf life. This alters the taste, texture and appearance of the fruit. Reduction in spoilage could expand the market for farmers in developing countries. However, there is sometimes a lack of understanding by researchers in developed countries about the actual needs of prospective beneficiaries in developing countries. For example, engineering soybeans to resist spoilage makes them less suitable for producing tempeh, a significant source of protein that depends on fermentation. Modified soybeans produce tempeh which chefs find lumpy, less palatable, and less convenient. This is much the same as certain varietals of apples which have been bred for appearance and often lack the taste qualities of less visually attractive varietals.
The first genetically modified food product was a tomato which was transformed to delay its ripening. Researchers in Indonesia, Malaysia, Thailand, Philippines and Vietnam are currently working on delayed-ripening papaya in collaboration with the University of Nottingham and Zeneca.
Biotechnology in cheese production: enzymes produced by micro-organisms provide an alternative to animal rennet – a cheese coagulant – and an alternative supply for cheese makers.
About 85 million tons of wheat flour is used every year to bake bread. By adding an enzyme called maltogenic amylase to the flour, bread stays fresher longer. Assuming that 10–15% of bread is thrown away as stale, if it could be kept fresh another 5–7 days then perhaps 2 million tons of flour per year would be saved. Other enzymes can cause bread to expand to make a lighter loaf, or can alter the loaf in a range of ways.
- Reduced dependence on fertilizers, pesticides and other agrochemicals:
Most of the current commercial applications of modern biotechnology in agriculture are on reducing the dependence of farmers on agrochemicals. For example, Bacillus thuringiensis (Bt) is a soil bacterium that produces a protein with insecticidal qualities. Traditionally, a fermentation process has been used to produce an insecticidal spray from these bacteria. In this form, the Bt toxin occurs as an inactive protoxin, which requires digestion by an insect to be effective. There are several Bt toxins and each one is specific to certain target insects. Crop plants have now been engineered to contain and express the genes for Bt toxin, which they produce in its active form. When a susceptible insect ingests the transgenic crop cultivar expressing the Bt protein, it stops feeding and soon thereafter dies as a result of the Bt toxin binding to its gut wall. Bt corn is now commercially available in a number of countries to control corn borer (a lepidopteran insect), which is otherwise controlled by spraying (a more difficult process).
Crops have also been genetically engineered to acquire tolerance to broad-spectrum herbicide. The lack of herbicides with broad-spectrum activity and no crop injury was a consistent limitation in crop weed management. Multiple applications of numerous herbicides were routinely used to control a wide range of weed species detrimental to agronomic crops. Weed management tended to rely on preemergence, herbicide applications were sprayed in response to expected weed infestations rather than in response to actual weeds present. Mechanical cultivation and hand weeding were often necessary to control weeds not controlled by herbicide applications. The introduction of herbicide-tolerant crops has the potential of reducing the number of herbicide active ingredients used for weed management, reducing the number of herbicide applications made during a season, and increasing yield due to improved weed management and less crop injury. Transgenic crops that express tolerance to glyphosate, glufosinate and bromoxynil have been developed. These herbicides can now be sprayed on transgenic crops without inflicting damage on the crops while killing nearby weeds.
From 1996 to 2001, herbicide tolerance was the most dominant trait introduced to commercially available transgenic crops, followed by insect resistance. In 2001, herbicide tolerance deployed in soybean, corn and cotton accounted for 77% of the 626,000 square kilometres planted to transgenic crops; Bt crops accounted for 15%; and "stacked genes" for herbicide tolerance and insect resistance used in both cotton and corn accounted for 8%.
- Production of novel substances in crop plants:
Biotechnology is finding novel uses beyond food. For example, oilseed can be modified to produce fatty acids for detergents, substitute fuels and petrochemicals. Potatoes, tomatoes, rice, tobacco, lettuce, safflowers, and other plants have been genetically engineered to produce insulin and certain vaccines. If future clinical trials prove successful, the advantages of edible vaccines would be enormous, especially for developing countries. The transgenic plants may be grown locally and cheaply. Homegrown vaccines would also avoid logistical and economic problems posed by having to transport traditional preparations over long distances and by having to keep them cold in transit. And since they would be edible, they would not need syringes, which are not only an additional expense in the traditional vaccine preparations but also a source of infections if contaminated. In the case of insulin grown in transgenic plants, it is well-established that the gastrointestinal system breaks the protein down therefore this could not currently be administered as an edible protein. However, it might be produced at significantly lower cost than insulin produced in costly bioreactors. For example, Calgary, Canada-based SemBioSys Genetics, Inc. reports that its safflower-produced insulin will reduce unit costs by over 25% or more and approximates a reduction in the capital costs associated with building a commercial-scale insulin manufacturing facility of over $100 million, compared to traditional biomanufacturing facilities.
- Animal biotechnology:
In animals, biotechnology techniques are being used to improve genetics and for pharmaceutical or industrial applications. Molecular biology techniques can help drive breeding programs by directing selection of superior animals. Animal cloning, through somatic cell nuclear transfer (SCNT), allows for genetic replication of selected animals. Genetic engineering, using recombinant DNA, alters the genetic makeup of the animal for selected purposes, including for producing therapeutic proteins in cows and goats. The FDA is considering for approval a genetically altered salmon with an increased growth rate.
- Criticism:
There is another side to the agricultural biotechnology issue. It includes increased herbicide usage and resultant herbicide resistance, "super weeds," residues on and in food crops, genetic contamination of non-GM crops which hurt organic and conventional farmers, etc. Besides this, there is the obvious discomfort and concern of the public concerned with preserving that which has developed on its own in nature for millions of years and seems to have a source of being many consider vital and original and which belongs, and which has superior taste with no equal in quality, character or suitability no matter how large or how small and which no man we know of could have ever invented or devised. In other words, the idea of leaving well enough alone has merit.
Biological engineering:
Biotechnological engineering or biological engineering is a branch of engineering that focuses on biotechnologies and biological science. It includes different disciplines such as biochemical engineering, biomedical engineering, bio-process engineering, biosystem engineering and so on. Because of the novelty of the field, bioengineer is still not clearly defined. However, in general it is an integrated approach of fundamental biological sciences and traditional engineering principles.
Biotechnologists are often employed to scale up bio processes from the laboratory scale to the manufacturing scale. Moreover, as with most engineers, they often deal with management, economic and legal issues. Since patents and regulation (e.g., U.S. Food and Drug Administration regulation in the U.S.) are very important issues for biotech enterprises, bioengineers are often required to have knowledge related to these issues.
The increasing number of biotech enterprises is likely to create a need for bioengineers in the years to come. Many universities throughout the world are now providing programs in bioengineering and biotechnology (as independent programs or specialty programs within more established engineering fields).
Bioremediation and biodegradation:
Biotechnology is being used to engineer and adapt organisms, especially microorganisms, in an effort to find sustainable ways to clean up contaminated environments. The elimination of a wide range of pollutants and wastes from the environment is an absolute requirement to promote a sustainable development of our society with low environmental impact. Biological processes play a major role in the removal of contaminants. Biotechnology takes advantage of the catabolic versatility of microorganisms and their ability to degrade/convert such compounds. New methodological breakthroughs in sequencing, genomics, proteomics, bioinformatics and imaging are producing vast amounts of information. In the field of Environmental Microbiology, genome-based global studies open a new era providing unprecedented in silico views of metabolic and regulatory networks. Environmental Microbiology also offers clues to the evolution of degradation pathways and to the molecular adaptation strategies to changing environmental conditions. Functional genomic and metagenomic approaches are increasing understanding of the relative importance of different pathways and regulatory networks to carbon flux in particular environments and for particular compounds. They will certainly accelerate the development of bioremediation technologies and biotransformation processes.
Marine environments are especially vulnerable since oil spills of coastal regions and the open sea are poorly containable and mitigation is difficult. In addition to pollution through human activities, millions of tons of petroleum enter the marine environment every year from natural seepages. Despite its toxicity, a considerable fraction of petroleum oil entering marine systems is eliminated by the hydrocarbon-degrading activities of microbial communities. Particularly successful is a recently discovered group of specialists, the so-called hydrocarbonoclastic bacteria (HCCB).
Biotechnology regulations:
The National Institutes of Health (NIH) was the first federal agency to assume regulatory responsibility in the United States. The Recombinant DNA Advisory Committee of the NIH published guidelines for working with recombinant DNA and recombinant organisms in the laboratory. Nowadays, the agencies that are responsible for the biotechnology regulation are: US Department of Agriculture (USDA) that regulates plant pests and medical preparation from living organisms, Environmental Protection Agency (EPA) that regulates pesticides and herbicides, and the Food and Drug Administration (FDA) whose responsibility it is to ensure that the food and drug products are safe and effective.
Education:
In 1988, after prompting from the United States Congress, the National Institute of General Medical Sciences (National Institutes of Health) (NIGMS) instituted a funding mechanism for biotechnology training. Universities nationwide compete for these funds to establish Biotechnology Training Programs (BTPs). Each successful application is generally funded for five years then must be competitively renewed. Graduate students in turn compete for acceptance into a BTP; if accepted, then stipend, tuition and health insurance support is provided for two or three years during the course of their Ph.D. thesis work. Nineteen institutions offer NIGMS supported BTPs. Biotechnology training is also offered at the undergraduate level and in community colleges.
REFERENCE:














0 comments:
Post a Comment